|
Common Name: Tersa Sphinx
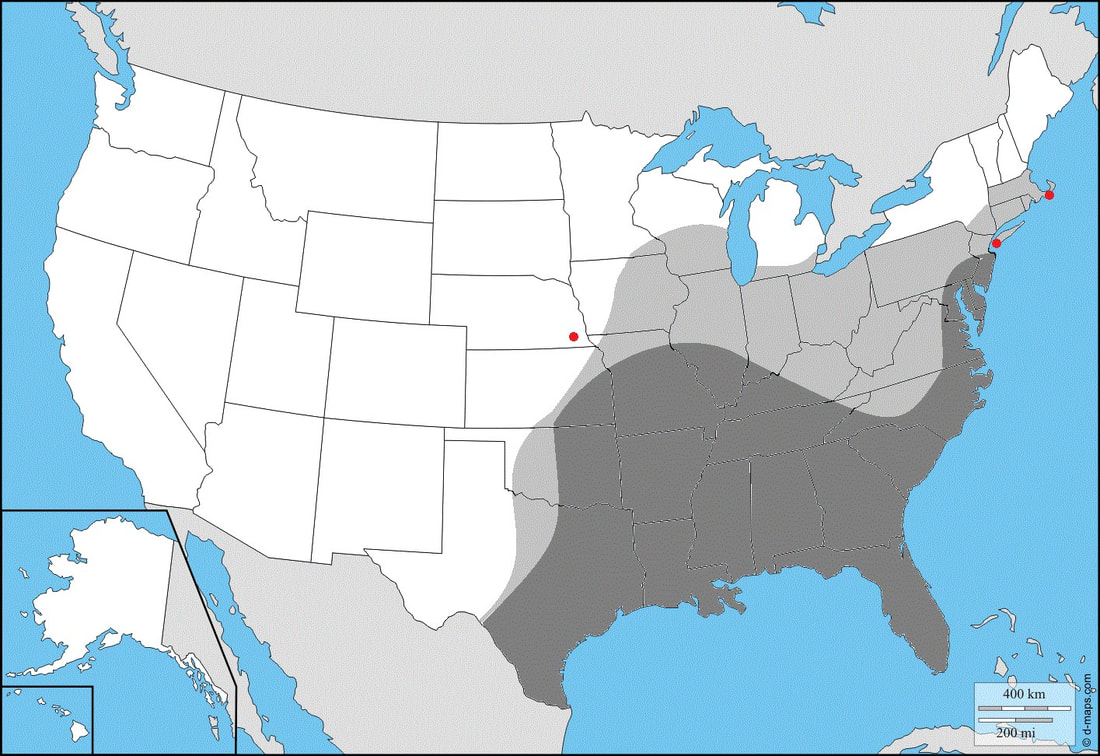
Ecology and Life History: This moth is active throughout the growing season, and can be found on the wing from May to November. In the deep south, this moth is active year-round. This species is quite attracted to artificial light, both sexes will come to light with males being more common. This is another Sphingidae species that likes to nectar on plants and can often be seen doing so, they do not seem to respond to bait. Both sexes of this moth look the same, females on average are larger, rounder, and have a noticeably thicker and rounder abdomen. In comparison, males are much more angular with a thinner abdomen. Eggs are laid singly on the leaves of hostplants. They are often deposited on the undersides of leaves near the tips. Young caterpillars tend to feed toward the tops of the plants, with larger final instars being found lower down and deeper into the plant. Due to the nature of these larvae to wander around the plant or to neighboring plants, it is not unlikely to find small or large caterpillars anywhere on the plant. This larva can have a green form or a brown form. Habitat and Searching for Larvae: This species is often found on Pentas lanceolaria, but also feeds on other plants in the Rubiaceae. Younger instar larvae are often spotted feeding at the tops of plants, sometimes within the flower clusters of Pentas (not feeding on the flowers though). Older larvae are a bit more subtle in their placement, being lower and deeper in the plants. All instars can be found relatively easily. This larva is fond of wandering to neighboring plants, particularly if there are many Pentas or similar hosts in a cluster. Due to this, it is not uncommon to find multiple instars on the same plant all feeding in different locations. This species is not gregarious and does not feed in groups. This is a species of suburban yards, it flourishes in these areas due to the high concentration of hostplants like Pentas. They are often seen nectaring and laying eggs on hostplants at dusk. Larvae can be found from May to November throughout most of their range, larvae can be found year-round in the Deep South. The larvae of this species fluoresce quite brightly under UV light making them easy to spot. Rearing Notes: Eggs are easy to obtain from wild females. Place the female in a screen cage with host material and food and she will likely deposit a number of eggs. Larvae are easy to rear on living hostplants but are a bit harder on cut hostplant. They will accept cut hostplant, but it must be fresh and replaced every other day. They are rather polyphagous, eating any of their known hostplants, most of the rearing done here has been using Pentas lanceolaria (1). The larvae do not seem to suffer diseases or deaths in captivity, especially when rearing in a screen enclosure on live hostplant. They are not sensitive to crowding when being reared on living host. If they’re being reared in tupperware, younger larvae (L1-2) can be kept fairly densely, but older larvae must be kept separate. They do not tolerate excessive humidity well and their enclosures must be kept clean. Sleeving is a perfectly good way to rear these larvae, care must be taken when they reach the prepupal stage as they will attempt to cocoon at the bottom of the sleeve. Unlike many Sphingidae, pupation occurs in a loosely spun silken cocoon on the soil surface or just under it. They will often use dried leaves or other plant debris in their cocoon. The pupa is loosely situated within this cocoon and can be easily removed. Host plants: Click here to load this Caspio Cloud Database
Cloud Database by Caspio |
Adult Description: This is another distinctive moth. The forewing length is 32-36mm (2). It is brown in color with very thin forewings. There are brown streaks on the forewing, the most obvious run diagonally from the apex of the wing to the base of the wing. The thorax is brown with orange and white parallel lines on either side. The abdomen is bicolored, brown on the dorsal surface and yellow on the sides. The hindwing is black with various amounts of yellow triangles on the bottom edge. The anal angle is often totally yellow. The yellow triangles on the hindwings separate this species out from Xylophanes libya found in Florida.
Larval Description: L1: This is a small green hornworm with a black horn. L3: At this stage the larva develops the defining markings needed for ID. The enlarged first few abdominal segments have a fake eyespot on them. There is a pale streak that runs through this eyespot and down the sides of the larva intersecting with 6 additional smaller spots. L5: The larva is generally green or brown, but can be much darker in color. There is a distinctive set of eyespots on the thoracic segment, followed by six more pairs on the abdominal segments. These abdominal eyespots are absent in Xylophanes pluto. The horn is black. |
The gallery to the left contains photos of Xylophanes tersa adults. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Xylophanes tersa larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Xylophanes tersa larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
|
|
|