|
Common Name(s): Gallium Sphinx; Bedstraw Hawkmoth
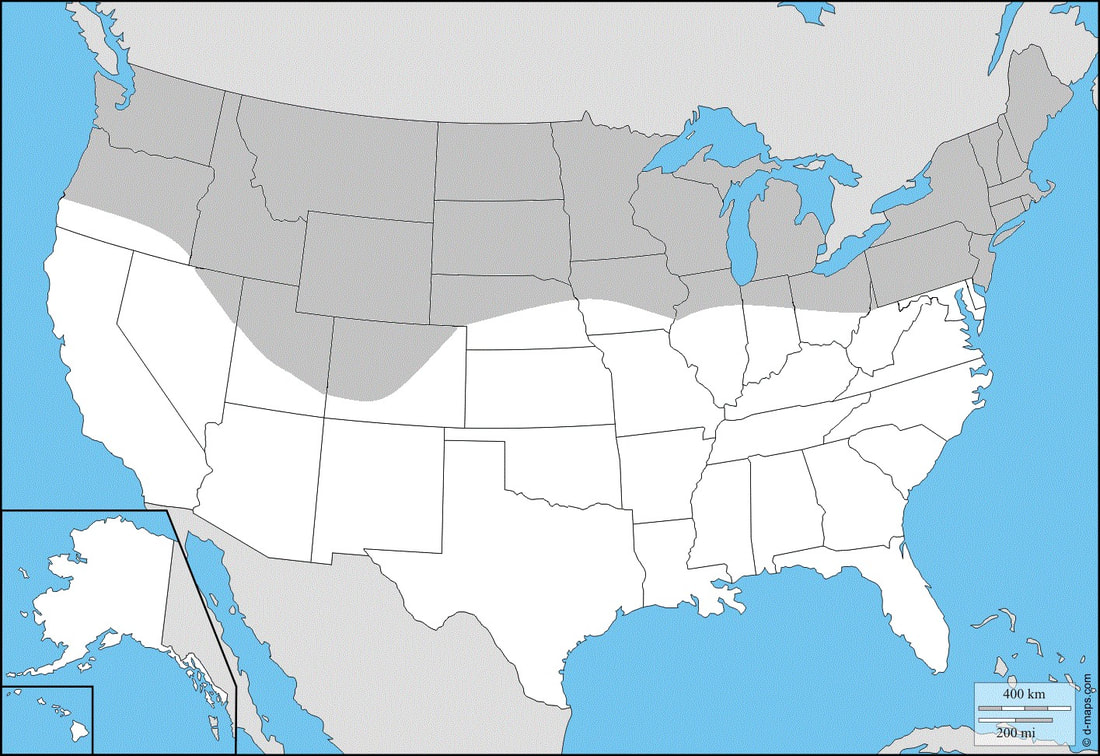
Ecology and Life History: This moth is active from June through September across most of its range. It can have up to 2 generations per year. This species is attracted to light, though usually not many will come into an artificial light at a time. Bait is an ineffective method for attracting this species, though searching flowers during the daytime and at dusk can be a good way to find this species' feeding. This species is not sexually dimorphic, with males and females being identically patterned. Males of this species are smaller and have slightly thinner abdomens. Females are often a bit larger, and have a more round abdomen. Sexing adults of this species can be quite hard, close examination of the genital region is often needed. Eggs are laid in small clusters on the leaves and stems of hostplants. Eggs tend to be laid toward the tops of the plants, or at the tips of the leaves. Young larvae feed on the undersides of the leaves, hiding along the mid-vein. Older larvae often descend the plant during the day to hide. Habitat and Searching for Larvae: The larvae of this moth eat plants in the genus Oenothera, Gallium, and Chaemaerion. As none of these plants ever get very tall, larvae can be found virtually anywhere on them or near them. Older larvae have a tendency to wander long distances before pupation, but also when their hostplant has run out of food and they are in search of more. Fields, roadsides, suburban yards, parks, forest edges, river edges, and meadows are good places to start looking for this species. Larvae are present from July through October, but are much more common toward the later half of the year when larvae are looking for places to pupate. The younger instars of this species fluoresce under UV light. The older, darker larvae do not fluoresce as well, but in some forms, the white or cream splotching fluoresces brightly. Rearing Notes: Eggs can be obtained by placing adults in a screen enclosure that is on it’s side. The enclosure should have a potted hostplant and an elevated nectaring dish (we use a petri dish with a 10% honey or sugar water solution on top of a tall mason jar. Placing flower petals in the dish helps accelerate feeding - fake or real petals work). Adults will fly both during the day and at night, feeding sparingly. Eggs are generally laid after the third day, and are laid for several nights afterward. One female can produce 200 eggs! This is a fairly polyphagous species, feeding on plants in the Onagraceae and Rubiaceae. They seem to be a bit pickier about their Rubiaceae, only consuming Gallium. It is worth trying other species of Rubiaceae. This species seems to be fairly hardy in captivity and doesn’t succumb to disease easily. Larvae do not seem to mind being crowded, and can be raised in quite dense conditions. The main concern with raising the larvae in high density is humidity. The humidity can build up quite rapidly in a closed container with 50+ larvae in it. While this species can handle bouts of high humidity, they do not like it. We recommend rearing larvae in a ventilated container, or aquarium with a glass lid. If you are rearing larvae in a sealed container, keep larvae in densities under 20 when they are final instars to avoid excess humidity build up. Pupation is easily achieved using the paper towel method (see the general information tab on this website). Once the larvae are prepupal and placed in the tupperware box, pupation usually occurs 5-9 days later. Pupae can quickly be removed from the box and stored for the winter, or placed in a rearing cage for eclosure. Host plants: Click here to load this Caspio Cloud Database
Cloud Database by Caspio |
Adult description:
This is a medium size moth with forewings length of 25-43mm (2). The ground color of the forewing is brown. There is a distinctive thick creamy streak that runs from just below the apex of the forewing to the bottom edge which is bordered by black. If you examine the moth closely, you will see that the thick creamy streak becomes quite thin, but does continue to the apex of the wing. In the similar Hyles euphorbiae, this creamy area is much broader at the apex of the wing. The hindwings are quite similar to Hyles euphorbiae in that they are black with a red band. The red band has a bit of white in the anal angle. Underneath the red band, there is a black area that borders the base of the wing. In Hyles euphorbiae, there is a much thinner black area and then more brown at the base of the hindwing. The thorax is brown with white border. There is a faint often dotted white line that runs the length of the abdomen. Larval description: L3: At this stage, the larvae are black with a black caudal horn. There is a broken yellow line above and below the spiracles on the thoracic and abdominal segments. In between this line is a variable amount of white speckling. L4: The larva can be either green or black. Both forms have noticeable yellow splotches in the subdorsal region, one splotch per segment. The horn is bicolored, red on the bottom with a black tip. At this stage, it looks remarkably similar to fourth instar Hyles lineata. L5: The larva is overall dark in color (black/brown), with one distinct cream colored spot on each abdominal segment. Some forms of this larvae may lack the cream spots on the abdominal segments, and instead be all black with white speckling. The horn is distinctly red in all forms. There is a patch the same color as the head (usually a shade of red) right behind the head capsule. Larva may have a white area around the spiracle, but this is more of a border rather than a second distinctive patch such as in Hyles euphorbiae. |
The gallery to the left contains photos of Hyles gallii adults. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hyles gallii larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hyles gallii larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
|
|