|
Common Name(s): Hummingbird Clearwing Moth
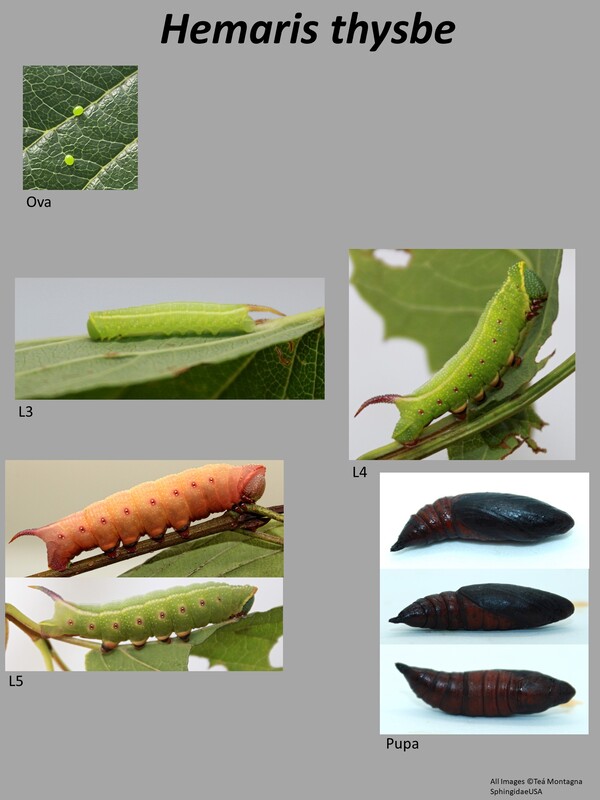
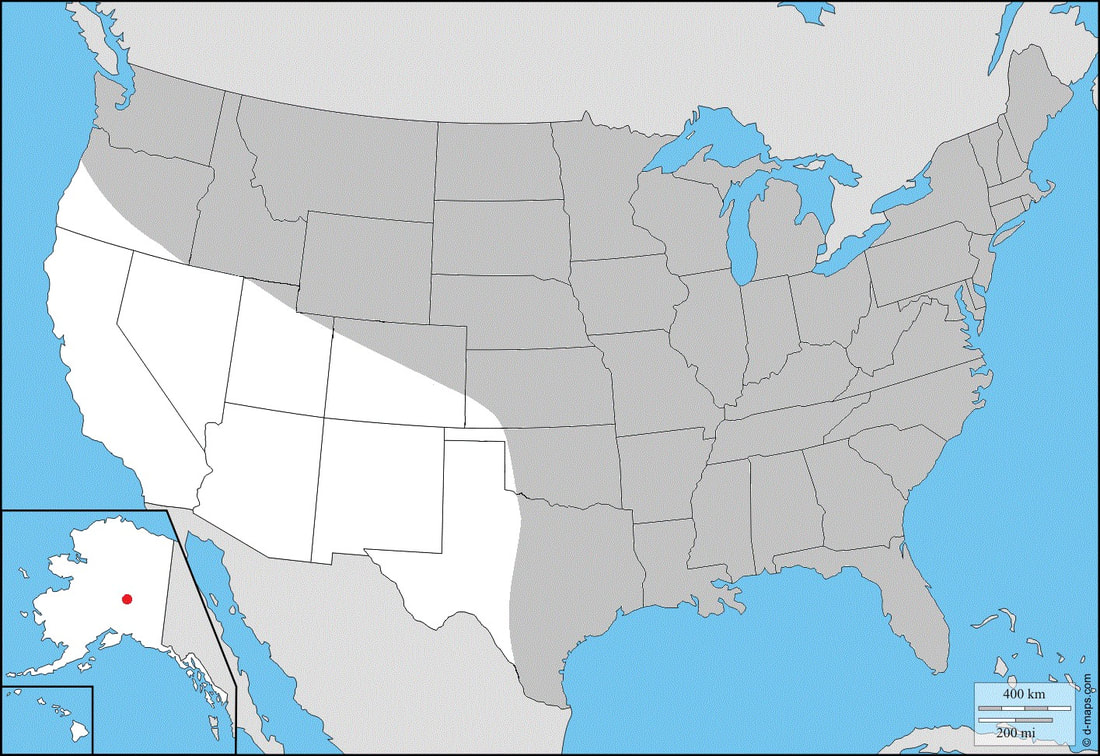
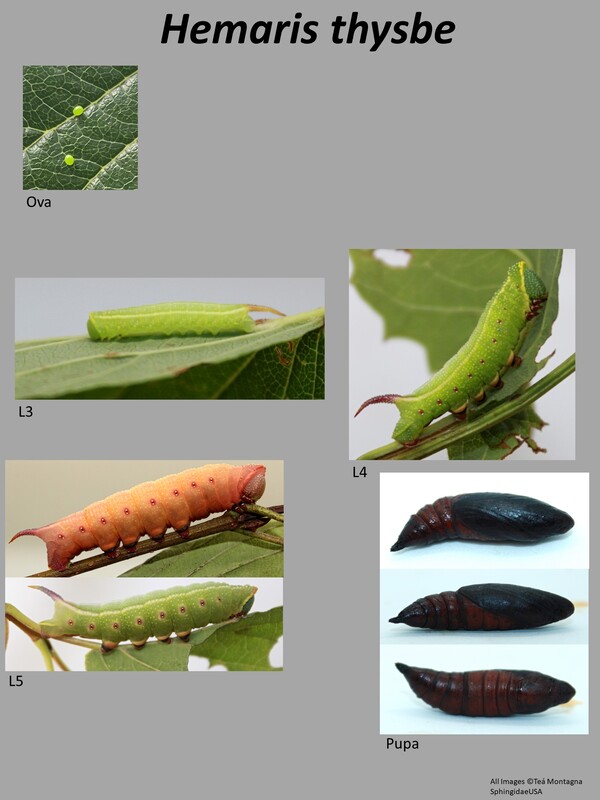
Ecology and Life History: This moth is active from May through September across most of its range. In some parts of the deep South, it may be present throughout most of the year. This is a diurnal species of Sphingidae and as such, it is not attracted to artificial lights set out at night. Instead, this species is most commonly seen taking nectar from flowers mid-day. Males and females are not sexually dimorphic and have the same wing maculations. Males in general tend to be slightly smaller than females, and have a thinner more pointed abdomen. Females tend to be slightly larger and have a rounder, blunt-tipped abdomen. In most species of Hemaris, sexing the adults can be quite difficult when they’re flying. Eggs are laid singly on the leaves and stems of hostplants. Young larvae feed on the undersides of the leaves, hiding along the mid-vein. Older larvae feed conspicuously at night, and can often be found in the open. During the day, they may retreat to the lower stems toward the base of the plant. Older larvae come in two distinct color forms, a green form and a brown form. Sometimes, there are intermediate color forms observed like pink or yellow, though these are more commonly associated with pupation. Habitat and Searching for Larvae: Larvae seem to prefer Viburnum as a host, though they’ll also consume other plants. It is quite possible that records for this species on Lonicera are incorrect, and rather the larva was mis-identified as Hemaris diffinis. Larvae can be found virtually anywhere on the plant. We have had luck searching isolated Viburnum along powerline cuts and marshes. Young larvae tend to be on newer growth, though this is not a hard rule. This is a species that inhabits parks, yards, urban areas, marshes, wetlands, fields, fencerows, and edge habitats. It is quite ubiquitous. It is not often found in deep, dense forests. Larvae can be found from June through October across most of its range. In the Deep South, larvae may be present year-round. This species fluoresces brightly under UV light, making this a great way to survey for larvae. Rearing Notes: Eggs can be obtained from adult females with the proper setup. Adults need natural sunlight to pair and lay eggs. Keeping a female in a screen enclosure with a potted hostplant and a nectar source in bright sun will likely yield eggs. This is a species that prefers Viburnum over most other plants. We have had most luck finding and rearing this species on Viburnum dentatum over any other plants. This species is not highly susceptible to disease when rearing in captivity. When rearing a large number of larvae together, it is best to rear them in a screen cage to prevent humidity build up. When there is sustained high humidity, the larvae are much more likely to die. Crowding can be an issue in this species, we recommend not rearing larvae in tupperware in high densities (over 15 larvae). In a screen cage, this can be increased (30-40). When rearing on a sleeved plant, as many larvae as can fit on the plant is usually ok. Pupation is easy to achieve using the Paper Towel Method (outlined on the general information tab of this website). Alternatively, a soil or soilless substrate can be used. Host plants: Click here to load this Caspio Cloud Database
Cloud Database by Caspio |
Adult description:
This is a small day-flying sphingid with forewing length of only 23-28mm (2). All the Hemaris species have clear areas on the wings rather than being typically colored and scaled like in other genera. This is a widespread species, found in many habitats. It can be fairly variable in appearance. The thorax is generally greenish-yellow or brown. There are two or so reddish segments on the abdomen, but the rest of the abdomen is the same color as the thorax. Tuttle notes that the lack of two distinct red bands that run down the ventral surface of the moth from eyes to abdomen which will help separate this moth out from Hemaris gracilis (2). You can also examine the forewing cell. In this species, the forewing cell has a distinctive vein running through the middle of it. Larval description: L5: The larva is green (or brown) in color and generally has a granulose yellow collar behind the head. The horn is bluish in color and granulose. The spiracles are red, but bordered by white, which helps separate it from Hemaris gracilis. There are two white stripes that run the length of the larva dorsally which end at the horn. The dorsal section of the larva between the white stripes may be more blue than the rest of the larva. |
The gallery to the left contains photos of Hemaris thysbe adults. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hemaris thysbe larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hemaris thysbe larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
|
|