|
NOTE: This species cannot be separated from Sphinx poecila without DNA barcoding.
See COMPLEX page for more information. Common Name(s): Apple Sphinx
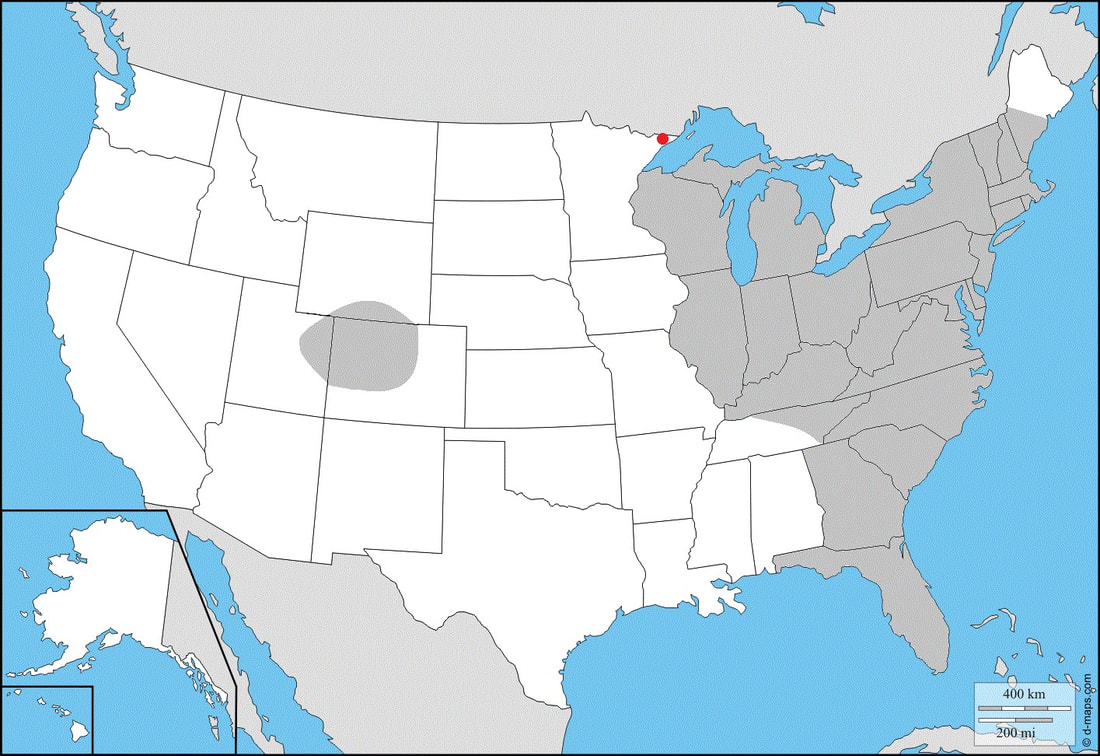
Ecology and Life History: This moth is active from May through July over most of its range. Both sexes are attracted to light, and good numbers will come in to a light when setup in the right habitat. Bait is not an effective method of attracting this moth. Both sexes of this species look identical. Males do tend to be slightly smaller, and their abdomens are thin, and come to a distinctive point. Females are slightly larger, and have a rounded abdomen that has a dull point, making them easier to identify which sex is which. Eggs are laid on the leaves of hostplants, usually on the underside. Young larvae feed on the undersides of leaves. Older larvae will hide on the stems and branches of plants, ascending to the leaves at night to feed. Habitat and Searching for Larvae: This species will eat a number of plants. Most commonly, Vaccinium and Comptonia in the Northeast. Larvae can be found virtually anywhere on the plant. Younger instars are most commonly found in the middle of the plant, on branches that stick out, feeding on the undersides of the leaves. Older larvae tend to hide deeper in the plant, not being as conspicuous. This is a moth of numerous habitats. We have recorded this species in Pine Barrens, Woodland Edges, Wetlands, Fens, and Fields. It does not seem to be a species that thrives in urban areas or near people. Larvae are reliably found from August to October in the Northeast. In the South, this species can be found from June to October. UV light is a great way of finding the larvae of this species as they fluoresce brightly. Rearing Notes: This species will readily lay eggs in captivity. Placing a female in a paper bag or styrofoam cooler will yield many eggs. Alternatively, placing a female in a flight cage with a potted hostplant is also a good way to get eggs. This species does well on a number of hostplants. In captivity, Vaccinium has been used to great success as it holds extremely well when cut. Comptonia or Malus may also be used, but both tend to wilt if left out of water or low humidity. This species can often succumb to diseases in captivity. It is not unheard of to lose an entire clutch to a viral or bacterial pathogen. Crowding this species is a good way to introduce lots of diseases into your clutch. We recommend rearing larvae in small quantities in tupperware, or slightly larger quantities in a screen enclosure. Larvae do not tolerate excess humidity well, and will die quickly when exposed to high levels of humidity. Sleeving is an excellent method of rearing this species. You do not need to worry about humidity levels, and crowding becomes less of an issue. When rearing larvae in a sleeve, keep only a moderate amount of larvae together as disease is still a worry. Pupation can be achieved by using the paper towel method (outlined in the general information tab), or by providing final instar larvae with a loose soil media. Host plants: Click here to load this Caspio Cloud Database
Cloud Database by Caspio |
Adult description:
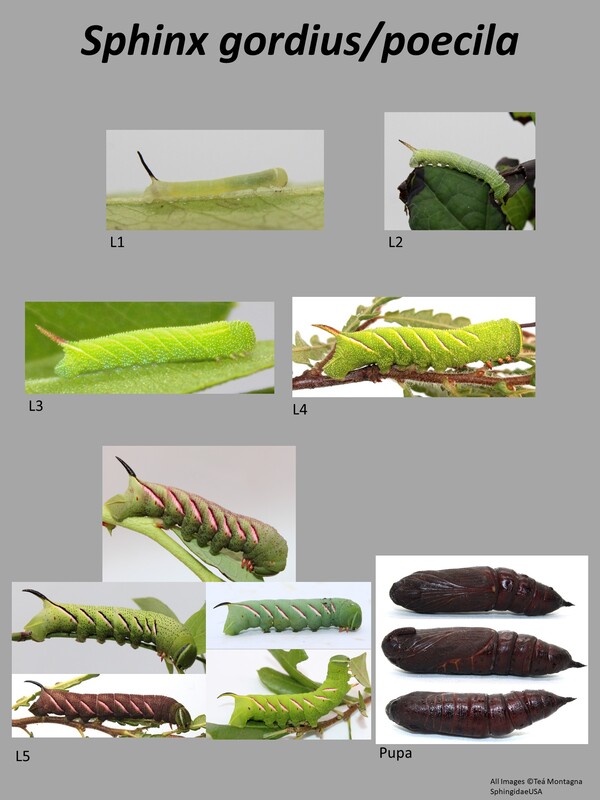
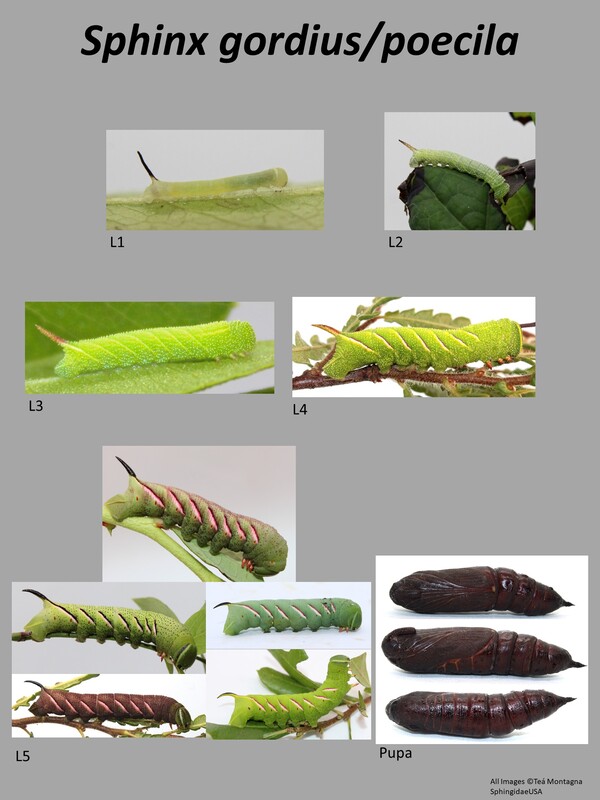
This is a medium sized (33-42mm forewing) moth (1). This species is often confused with Sphinx poecila, and they cannot be separated except by DNA barcode (COI gene). The general appearance of these two species is gray with black markings. There may or may not be a white discal spot. Larval description: L5: This species can be green or brown/black in color. There are 7 white lines on the abdominal segments. This and the larva of Sphinx poecila are seemingly identical and cannot be differentiated. |
The gallery to the left contains photos of Sphinx gordius adults. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Sphinx gordius larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Sphinx gordius larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.