|
Common Name(s): White Lined Sphinx
Ecology and Life History: This moth is active during the summer months (May-September) in the Northeast, but can be found year round in the Deep South. In the Southwest, this species is associated with the monsoon season, but can also persist year round in certain locales. This species is highly attracted to light, with both sexes showing up in good numbers. This species can also be seen nectaring at flowers at dusk or during the day. They do not seem to be attracted to bait. This species is not sexually dimorphic, both males and females look the same. Males tend to have a narrower more triangular abdomen, whereas females have a slightly rounder more cylindrical abdomen. Eggs are laid on the leaves and stems of plants. Larvae will feed practically anywhere on suitable hostplant, and have been known to wander to other nearby suitable hosts. This is one of our more variable caterpillars. The larva has about four different distinct forms (black, yellow-black, green, green-black), with different amounts of splotching or patterning. In some forms, the black may be reduced and the ground color may be more dominant. In other forms, the splotching of color may blend together and look like shading more than splotches. Habitat and Searching for Larvae: This species is incredibly polyphagous, and eats a wide number of plants. As such, it’s hard to pinpoint specific plants to search for finding this species. In the West, this caterpillar can become so numerous, roads must be shut down due to the amount of dead larvae on the road. This caterpillar is almost never out of reach, particularly because most of its hostplants are herbaceous plants. In the Northeast, Oenethera biennis (Evening Primrose) seems to be a favorite. This is a moth of many habitats, it can be found virtually anywhere, even in cities. It does not seem to be a denizen of deep forest habitats, but that is mostly due to lack of suitable hostplant. The green form of this caterpillar glows extremely well under UV light, whereas the black forms only have small reflective surfaces. Rearing Notes: Eggs can be obtained by placing a large sized mesh screen cage on its side and providing adults light, hostplant, and a nectar solution elevated off the ground (17). Adults will feed on their own, and will mate and deposit eggs with ease. This species eats a large number of things, and can be fed any one of its hosts. In captivity, our rearing has been on Evening Primrose (Oenethera biennis). Four O Clocks (Mirabilis jalapa) can also be used. Vitis and Parthenocissus quinquefolia have been used to achieve growth as well (17). This species is not prone to disease. High humidity is lethal to these larvae, even in a short period of time. If rearing in tupperware, vent the containers daily, if not twice daily. Do not overcrowd larvae in tupperware as it increases humidity to lethal amounts. In a mesh cage, large quantities of larvae can be reared easily on potted or cut host. The mesh cages also provide room for larvae to wander. Sleeving can be used, but mesh cages are superior. These larvae tend to want to wander from plant to plant, and a sleeve inhibits this. Additionally, larvae can chew out of sleeves. Pupation is easy to achieve using the paper towel method (outlined in the general information tab) or using soil. Host plants: Click here to load this Caspio Cloud Database
Cloud Database by Caspio |
Adult description:
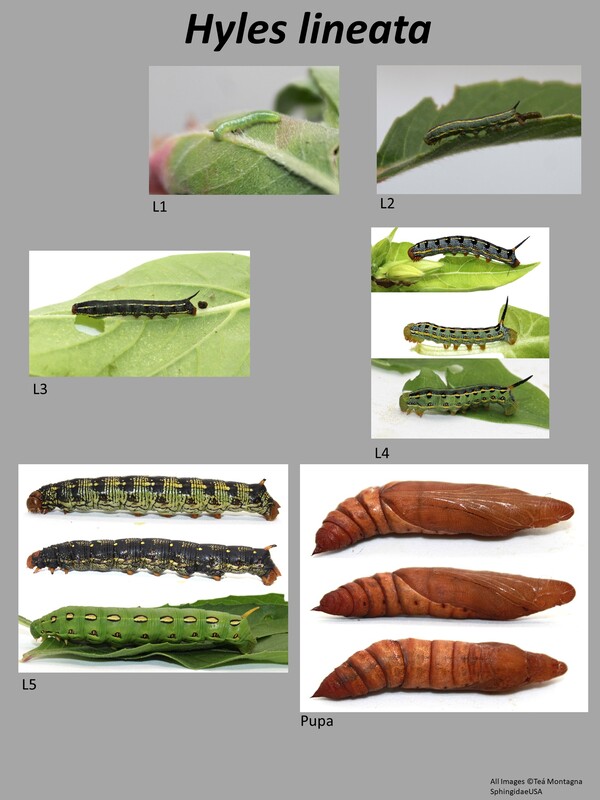
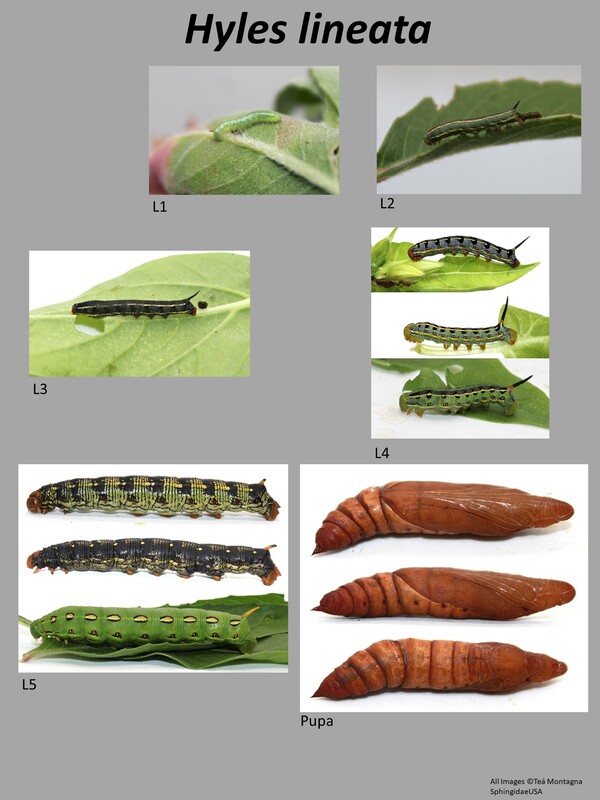
This is a medium sized moth with forewings 29-47mm in length (1). It is the most distinctive of the Hyles species in the US. The forewing is brown with distinctive white veining. There is a white-cream streak that runs from the anal angle of the forewing (where it is more white) to the apex of the wing (where it is more cream). The thorax is brown with lots of white streaking, easily separating it from the other species in this genus with plain thoraxes. The abdomen is quite striking: brown overall with white and black splotches with a white line down the middle. The hindwings are salmon colored bordered by black. Larval description: L5: The larva is highly variable, ground color can be green, brown, bluish, or black. There are usually several horizontal lines running the length of the larva: a dorsal line and two on either side. The larva itself usually has distinctive splotching along the side lines. The horn color is yellow or orange with some black on the tip. The head capsule is either green or yellow/orange. |
The gallery to the left contains photos of Hyles lineata adults. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hyles lineata larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hyles lineata larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
|
|
|