|
Common Name(s): Snowberry Clearwing
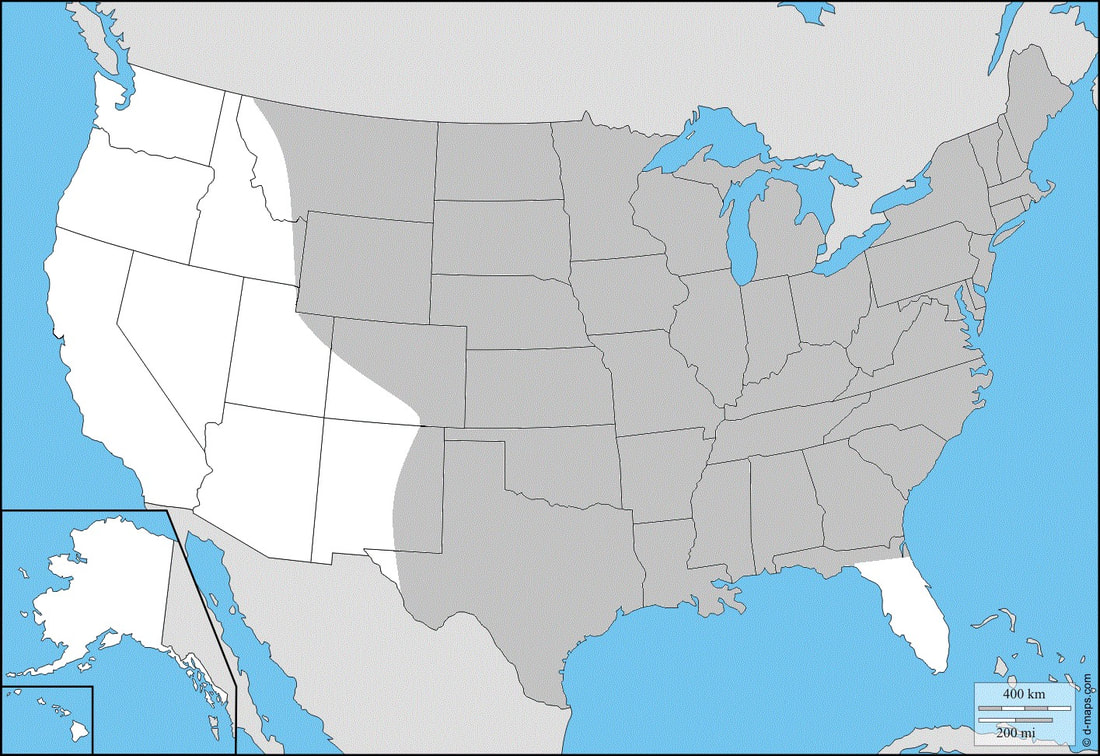
Ecology and Life History: This moth is active from April to October across most of its range. In the deep South, it may be present year-round. This is a day-flying species of Sphingidae, and as such is not attracted to artificial lights setup at night. The best way to locate this species is to go to suburban yards, parks, plant nurseries, and other areas where there are likely to be large amounts of flowers. Adults can be seen nectaring during the day from mid-morning to late-afternoon. Males and females of this species are identically marked and not sexually dimorphic. In general, males are slightly smaller and usually have smaller abdomens. Females are a bit larger, and have noticeably larger, rounder abdomens. This can be incredibly hard to tell in the field without examination of the live moth’s genital capsule. Eggs are laid singly or rarely in small clusters on the leaves and stems of hostplants. Young larvae hide along the undersides of the leaves along the mid-vein during the day, making small holes in the leaves. Searching for younger instars can be easily achieved by examining hostplants for small holes in the leaves and turning the leaf over. Older larvae are more difficult to find, sometimes hiding on the stems of the plant. The larvae of this species can come in green or black. The adults do not have regional forms. Habitat and Searching for Larvae: Larvae consume some plants in the Caprifoliaceae, specifically the Honeysuckle and Snowberry: Lonicera and Symphoricarpos. They will consume both the native species as well as the invasive species (Japanese Honeysuckle, and Japanese Bush Honeysuckle Complexes). Larvae can be found virtually anywhere on the plant. Young larvae are usually mid-height on the plant, or on branches that stick out from the rest of the plant. Searching new growth may also yield larvae. This moth can show up virtually anywhere. Fields, parks, yards, roadsides, and water edges are all good places to start looking for larvae. Larvae do not seem to mind urban areas, sometimes being found in the middle of cities next to major roadways. With the spread of several invasive Lonicera species, we expect this moth to become more prevalent and occupy additional habitats. Larvae are present from May through November across most of the range. These can sometimes be the last Sphingidae larvae present in the fall. The green form of this larva fluoresces brightly under UV light. The black form does not, however the spiracles will fluoresce a little, which makes finding this form manageable with UV light. Rearing Notes: This is a fairly easy species to obtain eggs from. Adults need natural sunlight to pair and lay eggs as well as a living hostplant. Placing adults in a large flight cage outdoor with food and a hostplant will almost certainly yield eggs. The larvae seem to prefer Honeysuckle or Snowberry in the lab. Interestingly, they will consume both the native and invasive Lonicera species without preference, often switching hostplants when offered. Reports of this species on Viburnum or Vaccinium are likely erroneous. Larvae are not too susceptible to disease, or sudden death when rearing. To ensure that populations are maintained, it’s best to not rear larvae in large densities as they are quite messy and the build-up of frass can be quite bad. We reccomend keeping 5 full grown larvae per medium sized tupperware container. Rearing can be easily done in a screen cage with potted or cut plants. Sleeving is another good method, but is only really reliable on large plants or the bush honeysuckles. You will want to sleeve a large portion of the plant with the larvae, and be prepared to move them at least once if you are rearing multiple. Pupation is easy to achieve using the Paper Towel Method outlined in the general information tab of this website. Host plants: Click here to load this Caspio Cloud Database
Cloud Database by Caspio |
Adult description:
This is a small day flying sphingid with forewing length of only 16-22mm (2). All the US Hemaris species have clear areas on the wings rather than being typically colored and scaled like in other genera. This moth resembles a bumblebee while flying, and the black and yellow coloration adds to the illusion. The abdomen of this species is primarily black with just a bit of yellow toward the tip. This helps differentiate it from the similar Hemaris thetis which is found only west of the rockies. To help determine species of Hemaris in the east, examine the forewing cell. If it is quite large and lacks a vein running through it, you have Hemaris diffinis or Hemaris aethra. In 2018 Chris Schmidt published a paper describing Hemaris aethra. This species is similar to Hemaris diffinis, but is typically more northerly in distribution. Hemaris aethra has much brighter red spotting on the forewing tips, and has yellow scales not interrupted by black in the center of the trailing abdominal scales (the scales that come off the tip of the abdomen). In Hemaris diffinis, the red spotting on the forewing is more brown in color, and the yellow portion in the center of the trailing abdominal scales is interrupted by black scales. Hemaris aethra is an obligate species on Diervilla lonicera, whereas Hemaris diffinis prefers Lonicera sp. Larval description: L5: The larva is green or black-brown and has very large distinctive black spiracles. The dorsal surface of this larva is slightly darker green than the rest. This is not evident in the brown form. Directly behind the head of this larva is a granulose yellow collar. The horn of this species is bicolored, yellow at the base and black the rest of the way and granulated. Hemaris aethra larva look very similar, except the spiracles are red. |
The gallery to the left contains photos of Hemaris diffinis adults. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hemaris diffinis larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
The gallery to the right contains photos of Hemaris diffinis larval and pupal stages. If you have a photo that you would like to submit to us, please contact us.
|
|